Stoichiometry And Problems
Stoichiometry is a section of chemistry
that involves using relationships between reactants and/or products in a
chemical reaction to determine desired quantitative data. In Greek, stoikhein means element and metron means
measure, so stoichiometry literally translated means the measure of
elements. In order to use stoichiometry to run calculations about
chemical reactions, it is important to first understand the
relationships that exist between products and reactants and why they
exist, which require understanding how to balanced reactions.
Balancing
In
chemistry, chemical reactions are frequently written as an equation,
using chemical symbols. The reactants are displayed on the left side of
the equation and the products are shown on the right, with the
separation of either a single or double arrow that signifies the
direction of the reaction. The significance of single and double arrow
is important when discussing solubility constants, but we will not go
into detail about it in this module. To balance an equation, it is
necessary that there are the same number of atoms on the left side of
the equation as the right. One can do this by raising the coefficients.
Reactants to Products
A
chemical equation is like a recipe for a reaction so it displays all
the ingredients or terms of a chemical reaction. It includes the
elements, molecules, or ions in the reactants and in the products as
well as their states, and the proportion for how much of each particle
is create relative to one another, through the stoichiometric
coefficient. The following equation demonstrates the typical format of a
chemical equation:
In the above equation, the elements present in the reaction are represented by their chemical symbols. Based on the Law of Conservation of Mass,
which states that matter is neither created nor destroyed in a chemical
reaction, every chemical reaction has the same elements in its
reactants and products, though the elements they are paired up with
often change in a reaction. In this reaction, sodium (Na
), hydrogen (
)
are the elements present in both reactants, so based on the law of
conservation of mass, they are also present on the product side of the
equations. Displaying each element is important when using the chemical
equation to convert between elements.
Stoichiometric Coefficients
In
a balanced reaction, both sides of the equation have the same number of
elements. The stoichiometric coefficient is the number written in front
of atoms, ion and molecules in a chemical reaction to balance the
number of each element on both the reactant and product sides of the
equation. Though the stoichiometric coefficients can be fractions, whole
numbers are frequently used and often preferred. This stoichiometric
coefficients are useful since they establish the mole ratio between
reactants and products. In the balanced equation:
we can determine that 2 moles of HCl
will react with 2 moles of Problem : What is the mass of 2 moles of H2S ?
GFM of H = 1
GFM of S = 32>br> GFM of H2S = 2×1 + 32 = 34 grams / mole
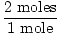 ×34 grams = 68 grams ×34 grams = 68 grams |
When 80 grams of aluminum is reacted with excess chlorine gas, how many formula units of AlCl3 are produced?
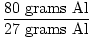 ×1 mole Al = 2.96 moles Al ×1 mole Al = 2.96 moles Al |
There is a 1:1 ratio between Al and AlCl3 , therefore there are 2.96 moles AlCl3 .
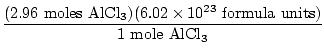 = 1.78×1025 = 1.78×1025
|
How to balance the reaction?
BalasHapusThe number of atoms between the two side must be equal or same. For example, having 2 Oxygen on one side and 2 on the other would be imbalanced
HapusWhy a chemical equation is like recipe for a reaction ?
BalasHapusbecause a chemical equation created from recipe a reaction, so to create a chemical equation we must know recipe of a reaction
HapusCan you explain about stoichiometry with the simple concept please?
BalasHapus@hudiahudhdu
stoichiometry is a branch of chemistry that studies the quantitative relationship of the composition of chemicals and their reactions. In stoichiometry we must know mole because mole is a center if we will find of volume, mass, particel, etc
HapusCan you explain about the difference about emprical formula and molecular formula?
BalasHapusThe molecular formula and the empirical formula are obviously very different, the difference is:
HapusThe molecular formula is a formula that states the number of elemental atoms that make up one molecule of a compound. Thus the molecular formula represents the actual arrangement of the substance molecule.
Example of molecular formula:
A. The water molecule formula is H2O which means that in one water molecule there are two hydrogen atoms and one oxygen atom.
B. C6H12O6 glucose molecule formula which means in one glucose molecule there are 6 carbon atoms, 12 hydrogen atoms, and also 6 oxygen atoms.
Differences Between Molecular Formulas and Empirical Formulas
2 Understanding the Empirical Formulas
The empirical formula is a formula that states about the smallest atomic ratio of the elements that make up the chemical compound.
Example of an empirical formula:
(A) Sodium chloride is an ionic compound composed of Na + and Cl ions with a ratio of 1: 1. The chemical formula of NaCl sodium chloride.
(B) Calcium chloride is an ionic compound consisting of Ca2 + ions and Cl - ions with a ratio of 2: 1. Chemical formula of calcium chloride CaCl2
BalasHapusIs there any benefit of stoichiometry in everyday life, if any please explain!
Its function is to:
Hapus1-Estimate the results of a reaction from a certain amount of preaksi,
2-Calculate how much material is needed, if desired a certain amount of reaction.
Its benefits in everyday applications: to determine the dosage in a process. For example: Dosage in making a food
Before we start chemical calculations, we equate the equation of the reaction first, how to equalize the equation of the reaction ?
BalasHapusEqualize the Reaction Equation
HapusThe basis of equating the reaction equation is the Law of Conservation of Mass (Lavoisier) namely: the amount of mass before the reaction equals the amount of mass after the reaction.
How to Equal Chemical Reactions:
1. Direct way (in simple reaction): equating the number of atoms in the left with right sides for each element, using coefficients (not index).
2.The way of separation (in complex reactions): the way of mathematics (substitution)
Problems example :
Consider the following reaction equation:
C2H60 + O2 → CO2 + H2O
Resolution:
Left side = right side
Atom C 2 = 2 x 1
Atom H 6 = 3 x 2
Atom O 1 + 3 x 2 = 2 x 2 + 3 x 1
The reaction equation becomes:
C2H6O + 3 O2 → 2 CO2 + 3 H2O